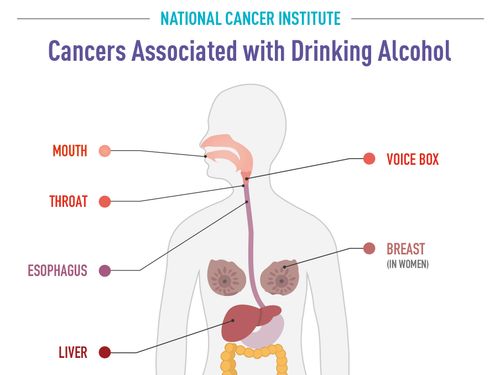
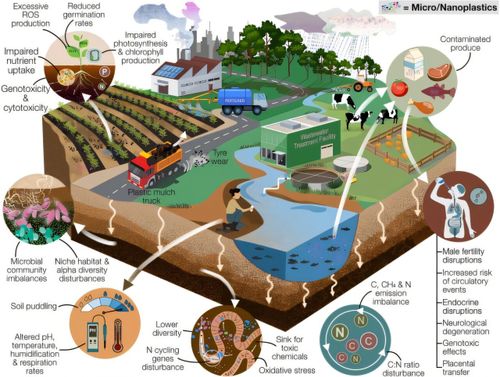
Moderna Withdraws Application For Flu-Covid Combo Vaccine After Consulting With FDA
Articles
Summary

Moderna Pulls Application for Flu-Covid Combo Shot in Setback
Moderna Inc. said it has “voluntarily” withdrawn its application for regulatory approval for its combination Covid and flu shot for people 50 and over, a setback for the company. The biotech company said it made the decision after consulting with the Food and Drug Administration. It plans to resubmit the application later this year after getting more data from a late-stage trial of its standalone flu vaccine, it said in a statement Wednesday.
Left
Bloomberg
Moderna pulls licensing submission for combo flu-COVID vaccine
Today vaccine maker Moderna announced it voluntarily pulled its licensing submission for the combination seasonal influenza–COVID-19 mRNA vaccine candidate, mRNA-1083, so that it can submit efficacy data. The news comes a day after the US Food and Drug Administration announced that seasonal COVID-19 boosters would now be recommended only for adults ages 65 and older or for those who are at risk for severe COVID-19 because of underlying health conditions. Moderna's combination vaccine was intended for adults 50 and older.
Middle
CIDRAPModerna Withdraws Combo COVID, Flu Shot Application
Moderna said on Wednesday it has withdrawn an application seeking approval for its flu and COVID combination vaccine candidate for adults aged 50 years and older. The company would resubmit the application later this year with vaccine efficacy data from a late-stage trial of its experimental seasonal influenza vaccine, it said. Moderna expects interim data from the trial to become available this summer. The company's decision comes a day after the U.S. Food and Drug Administration said it would require new clinical trials for approval of annual COVID-19 boosters for healthy people under 65 years.
Right
Newsmax
Pharmalittle: We’re reading about Trump’s drug-pricing plan, a Moderna change of plans, and more
The Trump administration shared the outlines of how it plans to push drug companies to lower their prices in the U.S.
Middle
STAT
Moderna withdraws application for COVID-flu combination vaccine
Moderna MRNA.O said on Wednesday it had withdrawn the pending application for its flu and COVID combination va
Middle
Jerusalem PostArticles
Summary
Articles
Summary
HEALTH
New Research Links Microplastics in Ultra-Processed Foods to Increased Risk of Depression and Dementia
Updated: 14 hours ago
Articles
Summary
HEALTH
RFK Jr.'s MAHA Report Paints Bleak Picture of Children's Health in the US
Updated: 20 hours ago
Articles
Summary